Chemical Properties of Candle Wax
In the demonstration burning of the candle is a chemical change while conversion of the candle to wax is a physical change. Speight 2014 2017 2019.

Melting Candle Wax To Explore States Of Matter States Of Matter Melting Candles Matter Science
The ingredients of the heat block.

. The burning of wax in a candle is a chemical change producing carbon carbon dioxide and water vapour. If too much collects and hardens it can pose a problem. Excess oil tends to pool in the candle if it contains poorly mixed fragrance or theres simply too much in the wax.
The properties of these starch products are determined both by their botanical origin and further chemical andor physical modifications. When a candle burns both physical and chemical changes occur. Spermaceti is a waxy substance found in the head cavities of the sperm whale and in smaller quantities in the oils of other whales.
Iron corrodes in salt water way faster than it does in water because electrochemistry is involved. Take a candle and light it. B ProcessB is a chemical change.
They then refer to their data table of chemical properties and use it to match a mystery substance or. C Both processes A and B are chemical changes. For example lavender is known for its calming properties while peppermint may enhance mental clarity.
Heat blocks melt snow layers and ice within 2 blocks taxicab distance. The combustion occurs as long as the fuel is available. Different wax and fragrance oil combinations may require more or less time to achieve an optimal state.
As time passes we can observe that the candle changes to wax. When a candle burns both physical and chemical changes take place. This organ may contain as much as 1900 litres 500 US gal of spermaceti.
These X-rays give scientists the ability to study the structural and chemical properties of matter. Record what you observe. There are some chemical reactions.
Melting of wax vapourisation of. Ii A chemical change cannot be easily reversed. Here the distinction can be made between the melting of the wax and the appearance of new materials.
Over the process heat and light energy is given out. Give another example of a familiar process in which both the chemical and physical changes take place. Chemical changes create a new product.
It is a chemical change. Either the mass is added or removed. The whaling industry in the 17th and 18th centuries was developed to find harvest and refine the.
The gas jar should be placed over the lit candle on a heatproof mat. If you cover the candle with a jar it will extinguish. Hsu and Robinson 2017It is a colorless and odorless oil that is used for varied purposes.
We do not get wax back from carbon carbon dioxide and water by cooling them or any other method. The mass of the substance is altered during a chemical change. Set up the apparatus as shown in the diagram.
Gary et al 2007. Paraffin oil or liquid paraffin oil is obtained in the process of crude oil distillation Parkash 2003. The wick of the candle gets changed to a black mass.
Granular native starch when heated in an aqueous environment gelatinizes to produce a viscous. Upon applying extremely high pressure several hundred atmospheres to. Prevent candle cracking and pull away.
It is unique in that it is the only block that melts objects without giving off light. A burning candle is the best example of physical and chemical change. It reveals that chemical change cannot be reversed by changing or altering the experimental changes.
During a chemical change energy changes occur. A starting activity could be observing the burning of a candle and discussing the changes that take place. PVC has an acrid smell like chlorine so stay away from the smoke and gas given off by PVC.
Speight PhD DSc PhD in Handbook of Industrial Hydrocarbon Processes Second Edition 2020 32 Paraffin oil. The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas because the wax is a solid water formed by the combustion of wax is a liquid at room temperature whereas carbon dioxide produced by the combustion of wax is a gas. How to pour NatureWax candles.
Again the wax combines with the atmosphere oxygen and changes to carbon dioxide heat and. LDPE and HDPE smell like candle wax while Polypropylene smells similar to candle wax but with an element of paraffin to it. The changes in chemical change are irreversible and permanent.
This change is not reversible. Earwax is a yellowish waxy material produced by the sebaceous gland in the ear canal. If it turns pink water is present.
Spermaceti is created in the spermaceti organ inside the whales head. D None of these processes is a chemical change. The gas evolved extinguishes a burning candle.
For example when we light a candle it will burn until the wax burns out. Hence the burning of a candle is a chemical change. The first industrially practical polyethylene synthesis diazomethane is a notoriously unstable substance that is generally avoided in industrial application was again accidentally discovered in 1933 by Eric Fawcett and Reginald Gibson at the Imperial Chemical Industries ICI works in Northwich England.
The following statements pertain to these changes. A ProcessA is a chemical change. Iii Change in state of substance.
A large amount of heat and light energy is produced in this type of reaction. To see exactly how theyre using SSRL to study the priceless documents watch the video above. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
When shopping for sustainable palm wax candles choose those that feature pure essential oils and plant-derived oils. State the differences between chemical and physical changes. Anaerobic bacteria digest animal waste and produce biogas ChangeA.
When the candle goes out put a lid on the gas jar. Heat some sugar crystals in a test tube over a flame. This means that this type of reaction does not need any external.
By firing the SSRLs thinner-than-a-human-hair X-ray beam at a block of text on a document researchers can create two-dimensional chemical maps that. Burning of candle melts the wax and hence physical state of wax has changed from solid to liquid. Essential oils instead of chemical fragrances Essential oils sit right at the heart of a candle that smells amazing.
Questions posed could include. This type of combustion occurs spontaneously. Test to see if the candle made water by adding a piece of blue cobalt chloride paper test the sides of the jar.
It is not possible to i recover the burnt wax again ii recover the thread again. Heat block is a block that can melt snow and ice just like a torch except it does not give off light. PET smells similar to burnt sugar the odour reminds the author of eating candy floss or sugar candy in his childhood.
Candle curing is the process of continuous hardening of wax to disperse fragrance oils evenly throughout the blend. The biogas is then burnt as fuel ChangeB.
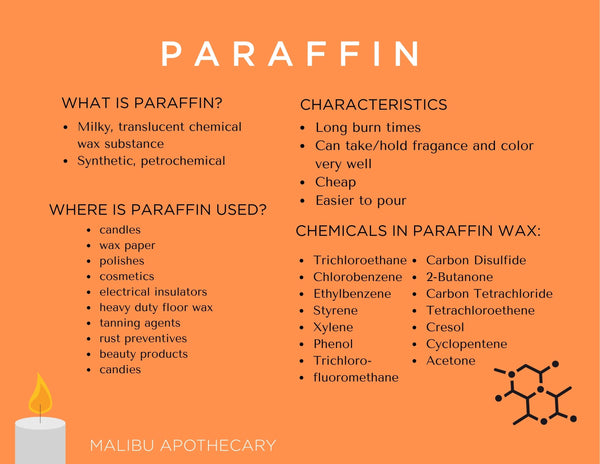
Is Paraffin Wax Toxic Malibu Apothecary
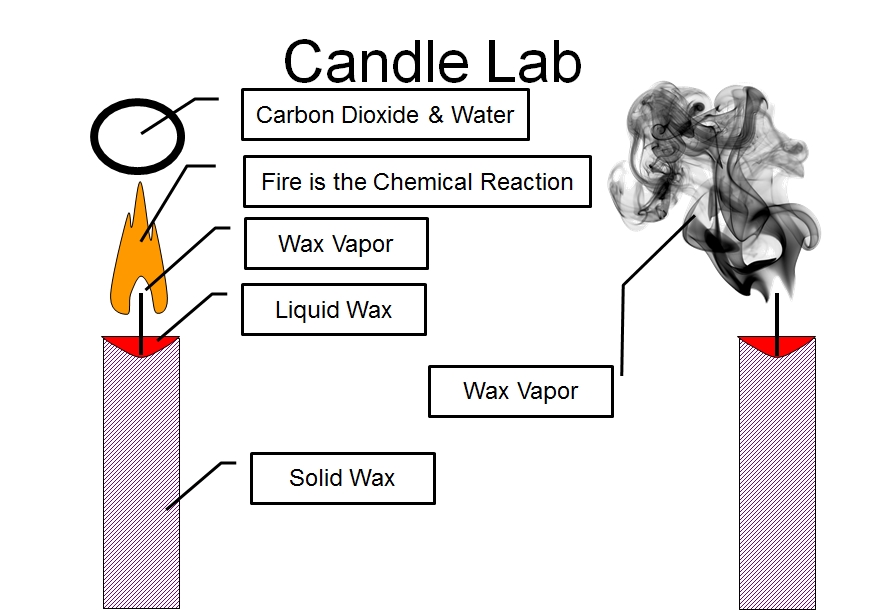
Candle Lab Vista Heights 8th Grade Science
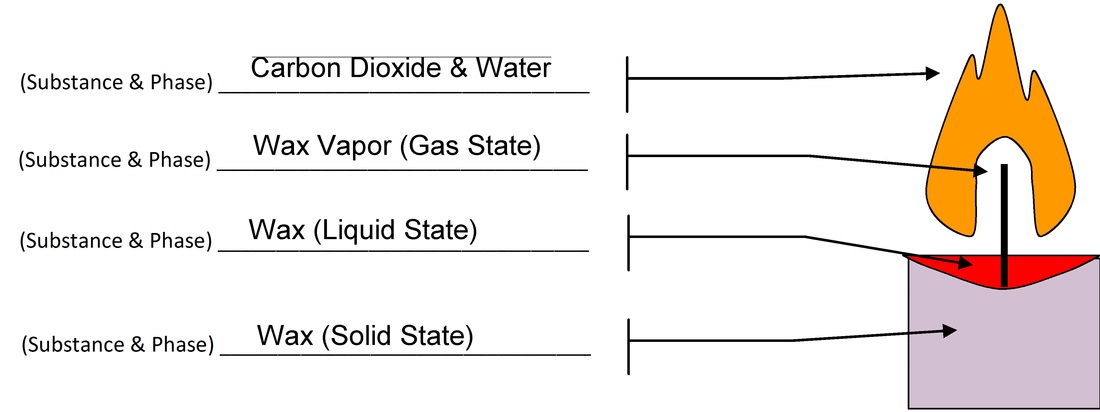
Candle Lab Vista Heights 8th Grade Science

What Is The Chemical Composition Of Candle Wax Lovetoknow
0 Response to "Chemical Properties of Candle Wax"
Post a Comment